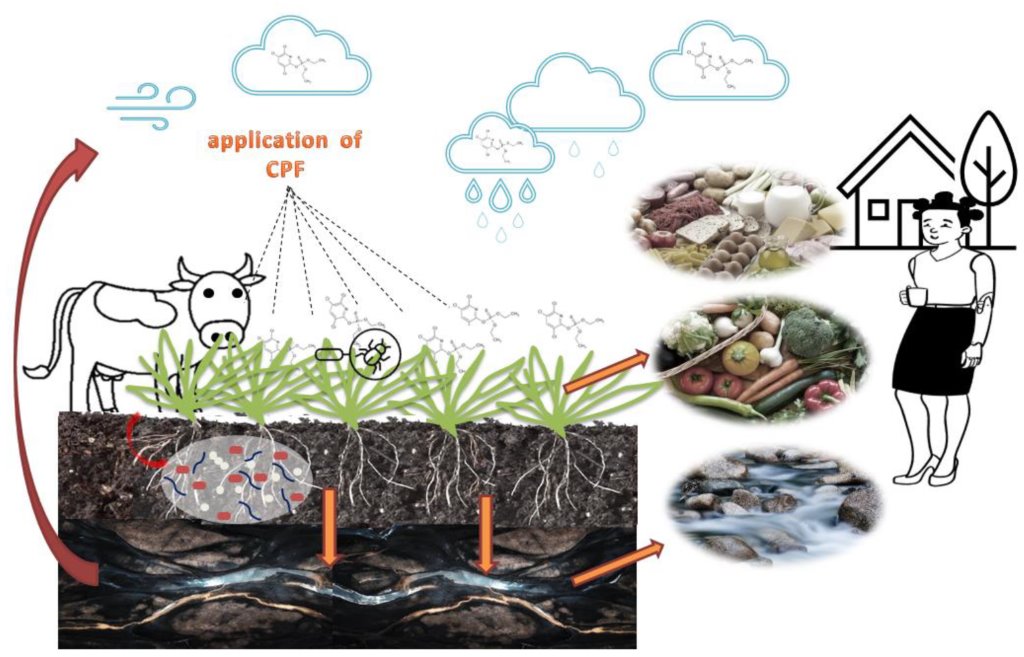
Chlorpyrifos is not an obscure chemical known only to scientists or regulators. For years, it has been one of the most widely used insecticides in modern agriculture, applied to crops that end up on everyday tables around the world. Fruits, vegetables, grains, dairy products, and even drinking water have all been found to contain traces of this pesticide. What makes chlorpyrifos especially troubling is not just how effective it is at killing insects, but how deeply it has embedded itself into the food system before its risks were fully understood.
Originally developed to target pests, chlorpyrifos works by disrupting the nervous systems of insects. That same mechanism, however, does not stop neatly at insects alone. As research expanded, scientists and health agencies began noticing patterns that raised uncomfortable questions about human exposure, especially during pregnancy and early childhood. Unlike acute poisonings that cause immediate symptoms, chlorpyrifos is associated with low-level, repeated exposure, the kind that can quietly accumulate over time without obvious warning signs.
As evidence grew, so did regulatory pressure. Scientific reviews, epidemiological studies, and toxicological experiments started pointing toward potential links between chlorpyrifos exposure and developmental neurological effects, reproductive concerns, and broader health risks. These findings triggered bans, restrictions, and heated debates across Europe and beyond, forcing regulators to weigh agricultural benefits against long-term public health consequences.
Understanding chlorpyrifos requires more than labeling it as “dangerous” or “banned.” It requires looking closely at how it is used, how people are exposed, what the science actually shows, and why authorities around the world reached very different conclusions about its safety. This article examines the pesticide’s widespread use, the scientific assessments behind regulatory decisions, and the health concerns that ultimately placed chlorpyrifos under intense global scrutiny.
How the hazard and risk assessment of chlorpyrifos-methyl was carried out
The hazard assessment of chlorpyrifos-methyl was initially conducted by examining its chemical structure and comparing it closely with chlorpyrifos. Because the two compounds share significant structural similarities, experts considered it reasonable to use existing knowledge about chlorpyrifos as a reference point. However, this approach also required careful attention to subtle molecular differences that could influence how each substance behaves in the body.
Although chlorpyrifos-methyl and chlorpyrifos are related, they do not produce identical toxic effects. Research has shown that chlorpyrifos-methyl exhibits higher acute toxicity, meaning it can cause harmful effects more quickly at lower doses. At the same time, both substances appear to share a similar level of toxicity when exposure occurs over long periods. This distinction is important, as it affects how short-term and chronic risks are evaluated for different populations.
In addition to the active ingredients themselves, experts also examined impurities present in the technical specifications of chlorpyrifos-methyl. Two toxicologically relevant impurities were identified, but available data suggested that these substances were unlikely to contribute additional hazards beyond those already associated with the parent compounds. Regulatory authorities reviewed the proposed maximum impurity levels and found them to be consistent with safety requirements, indicating that these impurities did not significantly alter the overall hazard profile.
Hazard assessment focuses on identifying the inherent properties of a chemical that make it potentially harmful, independent of how or where exposure occurs. This differs from risk assessment, which considers both the hazard and the likelihood of real-world exposure. Understanding this distinction is critical when evaluating pesticides like chlorpyrifos-methyl, as a substance can be highly hazardous in principle but present varying levels of actual risk depending on how it is used and controlled.
Main findings from the EFSA safety evaluation of chlorpyrifos-methyl
After completing a detailed review of the available scientific evidence, the experts from the European Food Safety Authority outlined several key conclusions regarding the safety of chlorpyrifos-methyl. These conclusions were based on toxicological studies, laboratory experiments, and epidemiological data, with particular emphasis on areas where uncertainties or data gaps existed.
One of the primary areas examined was genotoxicity, which refers to a substance’s ability to damage genetic material within cells. Although the studies available at the time did not clearly demonstrate genotoxic effects, EFSA experts emphasized that the overall dataset was limited. Because of this lack of comprehensive evidence, it was not possible to confidently rule out genotoxic potential for either chlorpyrifos or chlorpyrifos-methyl. At the same time, long-term animal studies involving rats and mice did not show evidence of carcinogenic effects following exposure to chlorpyrifos-methyl. While this finding was somewhat reassuring, it did not eliminate broader concerns related to genetic damage, particularly given the incomplete nature of the data.
Developmental neurotoxicity emerged as a more serious and well-supported concern. Studies involving chlorpyrifos showed neurological effects occurring at relatively low doses, especially during early stages of development. These effects are particularly troubling because they suggest that exposure during pregnancy or early childhood could interfere with normal brain development. The primary mechanism identified involves interference with cholinesterase, an enzyme that plays a critical role in the metabolism of choline, a key neurotransmitter involved in nerve signaling and brain function.
Although direct developmental neurotoxicity data for chlorpyrifos-methyl were less extensive, EFSA adopted a precautionary and conservative approach. Given the structural and toxicological similarities between the two compounds, experts applied the same concerns observed for chlorpyrifos to chlorpyrifos-methyl. This approach was considered necessary to protect public health in the absence of definitive evidence proving safety. In contrast, reproductive toxicity studies conducted over two generations in rats did not show adverse effects on reproductive performance, even at the highest tested doses. This finding suggested that reproductive capacity itself was not significantly impaired, although it did not address neurological outcomes in offspring.
Another important area reviewed was the potential impact on the immune system. In this case, EFSA identified a significant data gap. No adequate information had been provided to assess whether chlorpyrifos-methyl could cause immunotoxic effects. The absence of data prevented experts from drawing conclusions, adding another layer of uncertainty to the overall safety assessment.
Taking all these factors into account, EFSA concluded that the approval criteria related to human health, as defined under European pesticide regulations, were not met. The unresolved concerns regarding developmental neurotoxicity, combined with uncertainties surrounding genotoxicity and immune effects, were considered critical areas of concern. On this basis, EFSA advised against renewing the approval of chlorpyrifos-methyl for use within the European Union .
Observed effects of chlorpyrifos-methyl on human health
Evidence drawn from epidemiological studies has raised particular concern about the effects of chlorpyrifos-methyl on human health, especially during early development. Data examining exposed populations suggest an association between exposure to chlorpyrifos-based compounds and adverse neurological outcomes in children. These findings apply to both chlorpyrifos and chlorpyrifos-methyl, reinforcing the view that their toxicological profiles overlap in meaningful ways.
While the European Food Safety Authority acknowledged certain limitations and uncertainties within the epidemiological data, experts determined that the overall weight of evidence could not be ignored. When combined with toxicological findings from laboratory studies, the data supported concerns related to developmental neurotoxicity. This raised alarms about potential effects on the developing nervous system during pregnancy and early childhood, periods when the brain is especially sensitive to chemical disruption.
Based on the available evidence, EFSA experts concluded that chlorpyrifos-methyl could reasonably be expected to meet the criteria for classification as toxic to reproduction under European chemical safety regulations. Specifically, the compound was considered likely to fall under classifications indicating potential harm to the unborn child. Such classifications reflect a high level of concern, as they are reserved for substances with credible evidence of developmental harm. Although EFSA provided the scientific assessment, the final decision regarding official classification rests with the European Chemicals Agency, which evaluates how substances are labeled and regulated within the European Union .
Health risks linked to spray drift exposure and pesticide residues in food
Beyond direct toxicological effects, regulators have also examined the risks associated with real-world exposure to chlorpyrifos through multiple pathways. In the United States, the Human Health Assessment Branch of the Department of Pesticide Regulation, operating under the California Environmental Protection Agency, conducted a detailed evaluation of potential exposure scenarios. This assessment considered dietary intake, inhalation of spray drift, skin contact during and after application, and combined exposure from multiple sources.
When individual exposure routes were evaluated separately, such as inhalation of airborne spray drift near treated fields or ingestion of residues through food, no immediate risks were identified for children or women of childbearing age. These findings suggested that isolated exposure pathways, when considered alone, might not exceed established safety thresholds.
However, a different picture emerged when regulators calculated aggregate exposure. This approach accounts for all possible routes of contact at the same time, including food consumption, drinking water, inhalation of airborne particles, skin contact with residues deposited on surfaces, incidental soil ingestion, and common hand-to-mouth or object-to-mouth behaviors. When these exposure sources were combined, the estimated margin of exposure fell below the target safety margin of 100. Such a result indicates that overall exposure levels could approach or exceed thresholds associated with harmful effects.
Adding to this concern, some scientific studies have suggested that neurological effects may occur at doses lower than those required to cause measurable inhibition of cholinesterase, the enzyme traditionally used as a marker of organophosphate toxicity. This raises questions about whether existing safety benchmarks fully capture the range of possible neurotoxic effects, particularly subtle or long-term impacts.
In parallel, EFSA evaluated the presence of chlorpyrifos-methyl residues in specific food products, including kaki, also known as Japanese persimmons, and pomegranates. Based on current data, EFSA concluded that both short-term and long-term dietary intake of residues from these sources, including exposure to the metabolite 3,5,6-trichloropyridinol, was unlikely to pose a significant risk to consumer health. This conclusion reflects average consumption patterns and regulatory residue limits, rather than worst-case exposure scenarios .